Abstract: The academic paper “Clinical Experiments and Results of the Application of Innovative Biophysics-based CDA Technology in Efficacy Evaluation of Lung Cancer” written by Anpac Biotech in collaboration with the School of Life Sciences of Fudan University and Shanghai Changhai Hospital was approved by the American Association for Cancer Research. (AACR) accepted, and will be presented and published as a poster at the 113th American Association for Cancer Research (AACR) Annual Meeting on April 12, 2022. This paper announced the latest important results of clinical verification of the Cancer Differentiation Analysis Technology (CDA) developed by Anpac Bio-Innovation for the evaluation of efficacy in lung cancer. Based on the correlation statistics and analysis of CDA detection data collected during treatment of 686 lung cancer patients and the clinical response (efficacy) of these lung cancer patients to lung cancer treatment, it was initially verified that CDA data and clinical response have a statistically good correlation. Sex, CDA technology is expected to become an effective and innovative method for efficacy evaluation in cancer treatment.

Recently, Anpac Biotechnology jointly presented and published a paper “Based on Innovative Biophysics” in the form of a poster at the 113th American Association for Cancer Research (AACR) Annual Meeting held from April 8 to 13, 2022. “Clinical Experiments and Results of CDA Technology Applied to Lung Cancer Therapeutic Response” (A Novel Bio-Physical Based CDA Approach to Lung Cancer Therapeutic Response). The co-authors of the paper are the National Key Laboratory team from the School of Life Sciences of Fudan University, a well-known Chinese university, and the excellent medical team from Shanghai Changhai Hospital.
 The 113th American AACR Conference – Anpac Biotech
The 113th American AACR Conference – Anpac Biotech
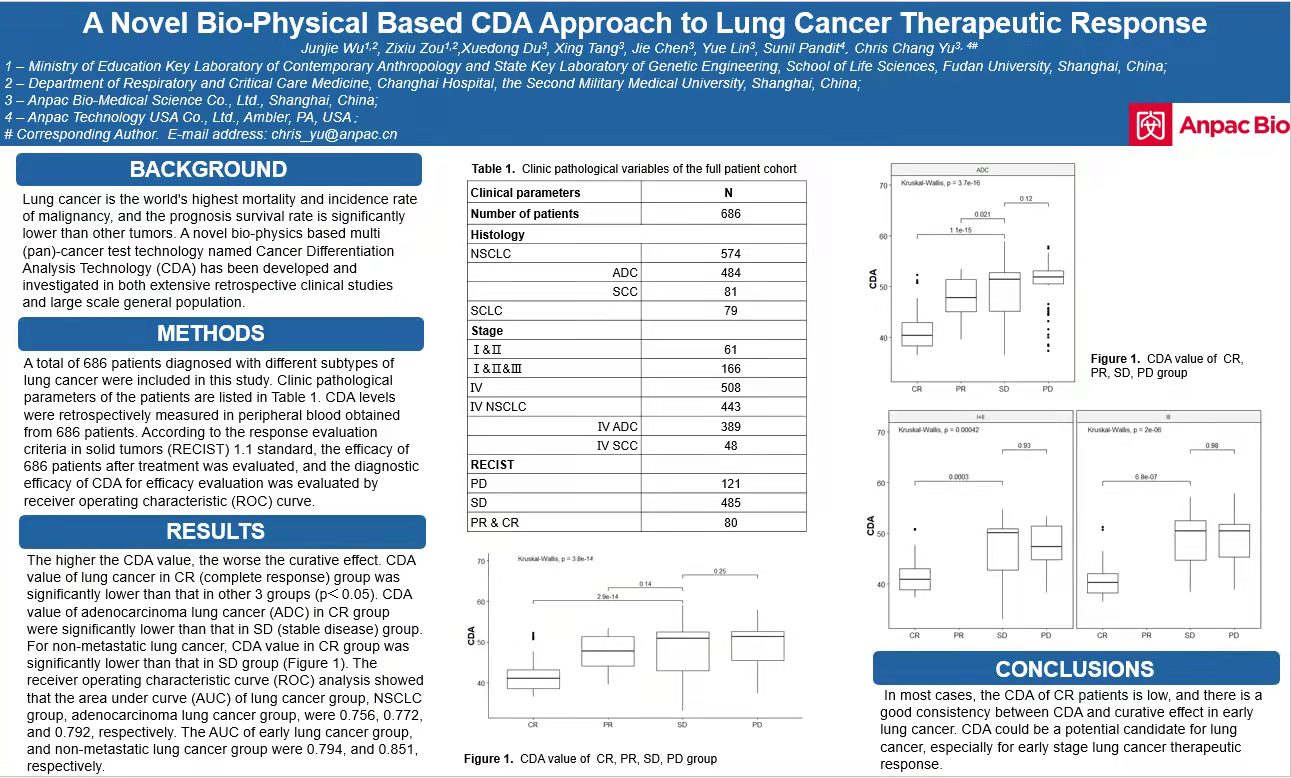
Lung cancer has the highest mortality and morbidity among malignant tumors in the world, and its prognosis and survival rate are significantly lower than other tumors. The research paper published this time announced the clinical experiments and results of the application of Anpac Biotech’s innovative CDA technology in the evaluation of the efficacy of lung cancer. It involved a total of 686 patients diagnosed with different types of lung cancer, and CDA detection of peripheral blood was taken. We have conducted clinical studies and evaluated the efficacy after treatment and found that:
1) The overall results show that the lower the CDA value, the better the efficacy (the higher the CDA value, the worse the efficacy);
2) The CDA value of the complete response (CR) ① group is significantly lower than the other 3 groups (partial response (PR) ②) stable disease (SD) ③, and progressive disease (PD) ④), and there is a statistically significant difference ( P value is lower than 0.05. In most cases, P value is much lower than 0.05);
3) The CDA value of adenocarcinoma lung cancer (ADC) in the complete response (CR) group is significantly lower than that in the stable disease (SD) group;
4) For non-metastatic lung cancer, the CDA value in the complete response (CR) group was significantly lower than that in the stable disease (SD) group.
The results showed that the CDA value in the complete response (CR) patient group was the lowest, and the CDA value in the early lung cancer group was consistent with the efficacy. CDA technology is expected to be one of the effective evaluation methods in the treatment of lung cancer (especially early-stage lung cancer).
The 113th American AACR Conference – Anpac Biological Paper Poster
Dr. Yu Chang, co-founder of Anpac Bio, said: “We are very pleased that Anpac Bio published the latest and important paper on the application of CDA technology in the efficacy evaluation of lung cancer at the AACR meeting. The results of this paper once again verified the CDA technology The clinical significance and the possibility that its application can be extended to the field of cancer efficacy evaluation. Once again demonstrates the feasibility and clinical value of our innovative biophysical approach in many aspects of cancer, and provides the possibility for CDA technology to be used in clinical cancer efficacy evaluation. and clinical value. We will continue to invest in research and development, accelerate the verification and transformation of our innovative results through industry-university-research cooperation, and continue to make positive contributions to the human fight against cancer.”
About AACR
The American Association for Cancer Research (AACR) was founded in 1907. It is the oldest and largest scientific organization dedicated to cancer research in the world. It currently has more than 48,000 members in 127 countries, including laboratories, Translational and clinical researchers; other health care professionals and cancer advocates; members include the AACR Academy’s 256 fellows; 54 Nobel Prize winners. The mission of AACR is to prevent and treat pain through research, education, communication, and collaboration. AACR’s scientific breadth and reputation for excellence attract researchers in the field, promote the exchange of new knowledge and ideas among scientists in the field of cancer research, provide training opportunities for the next generation of researchers, and increase public awareness of cancer.
About Anpac Biotechnology
Anpac Biotech is a biotechnology company focused on early cancer screening and detection as well as cancer treatment. As of September 30, 2021, it owned 150 patents. Anpac Biotech has two certified clinical laboratories in China and one CLIA and CAP accredited clinical laboratory in the United States. A range of cancer screening and detection tests are available, including CDA (Cancer Risk Assessment Technology), biochemical, immunological and genomic testing. A market research report by Frost & Sullivan shows that Anpac Biotech ranks first in the world in terms of sample volume for multi-cancer screening and testing (cumulative as of January 2021). These clinical experiments show that Anpac Bio’s CDA technology and platform can detect the risk of more than 20 types of cancer with high sensitivity and specificity.
Efficacy evaluation classification
① Complete response (CR, complete response): all target lesions disappear, no new lesions appear, and tumor markers are normal, lasting at least 4 weeks.
② Partial response (PR, partial response): The sum of the maximum diameters of target lesions is reduced by ≥30% and maintained for at least 4 weeks.
③ Stable disease (SD): The sum of the maximum diameters of target lesions shrinks to less than PR, or increases to less than PD.
④ Progressive disease (PD): the sum of the maximum diameters of target lesions increases by at least ≥20%, or new lesions appear.



