Shangguan News Report:
All cancer categories have relatively high specificity, above 95%, indicating that this blood testing technology is suitable for cancer screening in the general population.

Recently, the Clinical Research Center of Shanghai Jiao Tong University School of Medicine and Anpac Biotech, together with experts from the Children’s Hospital of Fudan University, Shanghai Tongren Hospital and other units, published an article introducing a new type of cancer in the international medical journal “Molecular Diagnostics Expert Review” The paper on screening methods provides a theoretical basis for the application of cancer risk assessment (CDA) technology in large-scale cancer screening, allowing multiple cancers to be detected early with low-cost blood tests, allowing patients to receive early treatment.
 Publish papers online
Publish papers online
Due to high costs and immature methods, routine health examinations currently often lack effective cancer screening tests. In response to this pain point, CDA technology has developed rapidly in recent years because scientific researchers have discovered that the biophysical properties of blood and related components are related to the occurrence of cancer, and can be detected through micromechanics, acoustics, optics, electricity, magnetism, etc. Identification using a variety of detectable biophysical properties.
In this cutting-edge field, Dr. Yu Chang, co-founder of Anpac Biotechnology, led the team to propose innovative methodologies and cancer detection concepts of “pan-cancer” and “multi-level, multi-parameter screening”, and independently developed a series of CDA technologies. They collect clinically significant weak signals at the protein level, cellular level and molecular level, and then conduct risk assessments on the existence and possibility of cancer through multi-parameter calculation model analysis. They are early, forward-looking, cost-effective, Appropriate sensitivity, relatively high specificity and other characteristics.
A paper published in Molecular Diagnostics Expert Review shows that researchers conducted a two-stage study to evaluate the value of CDA technology. The first phase of the cross-sectional study included 75,942 healthy individuals during routine health examinations; the second phase was a population-based prospective cohort study including 1,957 healthy community members. In a cross-sectional study and a prospective population-based cohort study, they found 48 and 10 cases of cancer, respectively. Since this study is a healthy population screening and prospective study, its specificity and sensitivity will be further improved as follow-up continues and more patients are diagnosed in medical institutions. Based on follow-up data as of June 30, 2020, researchers found that the CDA technology test can differentiate between healthy and cancer groups. Among the 75,942 healthy individuals in the first phase, based on 7,999 people who were successfully followed up, at the 95% confidence level, the specificity of CDA technology was greater than 95%, and the sensitivity was 66.7% for gastric cancer, 66.70% for liver cancer, and 62.50% for lung cancer. Overall, in both studies, the specificity was greater than 93% and the sensitivity was greater than 55%.
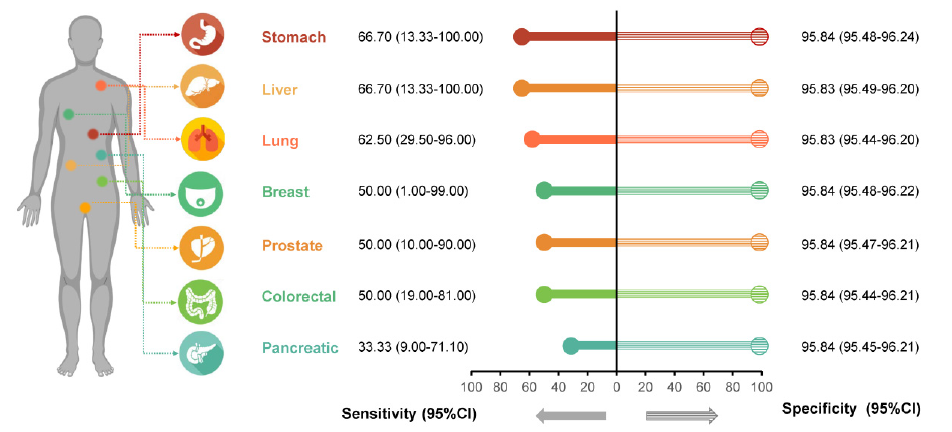 Application of CDA technology in follow-up (N=7999)
Application of CDA technology in follow-up (N=7999)
Among the 1,957 prospective cohort study participants in the second phase, 10 were diagnosed with cancer for the first time. Research shows that men in the 70-85 age group have higher CDA values, while women are less affected by age. The distribution in each age group is roughly the same, and the CDA value in 75-80 years old is slightly higher. Taking a CDA value greater than 48 as the critical point, the sensitivity and specificity of gastric cancer, liver cancer and lung cancer are relatively close.
Overall, all cancer categories have relatively high specificity, above 95%, indicating that this blood testing technology is suitable for cancer screening in the general population. At present, the CDA technology platform jointly developed by Anpac Biotech and medical institutions can detect the risk of more than 20 types of cancer with its high sensitivity and specificity.
Report source: Shangguan News



