Anpac Biomedical Technology Co., Ltd. (“Anpac Bio”), a company engaged in early detection of cancer, recently announced that it had received the first payment from a Malaysian customer at the end of February 2023. Biotech has previously signed an equipment purchase contract for the purchase of cancer detection equipment from Anpac Biotech, with a total amount of approximately 10 million yuan (US$1.5 million). The cancer detection equipment is developed and manufactured based on the company’s new multi-cancer detection patented technology – “Cancer Differentiation Analysis Technology” (hereinafter referred to as “CDA”). As the first important sales of Anpac’s cancer detection equipment in Southeast Asia, it is another milestone for Anpac. It marks that Anpac has officially entered the global cancer detection equipment market, and also confirms that Anpac has officially entered the global cancer detection equipment market. Biotech’s new technologies, reputation and ability to penetrate vast global markets. In addition to Malaysia, Anpac Biotech is also in business negotiations with relevant companies and institutions in many other countries.

According to the terms of the purchase contract, Anpac Biotech will provide cancer detection equipment as well as maintenance services, consumables and equipment parts to Malaysian customers. After the equipment is sold, the company will continue to generate ongoing revenue from the sale of services, consumables and equipment parts. The Malaysian client plans to establish a biomedical laboratory, obtain Laboratory Developed Test (LDT) certification, and perform cancer testing for a fee.
Anpac Bio’s CDA technology is based on measuring biophysical properties in blood. Compared with traditional technologies, it has several major advantages, including the ability to detect a variety of cancers at an early stage, while having low cost, good bioinformation security, relatively simple operation, and high sensitivity and specificity. According to a 2021 report by Frost & Sullivan, an authoritative U.S. market analysis and research company, Anpac Biotech ranks globally in terms of commercial sample size and total sample size for next-generation multi-(pan-) cancer early screening. First. At the same time, Anpac Biotech is continuing to conduct one of the world’s earliest and multi-year follow-up studies on multiple cancers with a large sample size (as of February 2023, more than 18,000 people have been enrolled in this follow-up study).
Dr. Yu Chang, co-founder of Anpac Biotech, said: “Outside of China and the United States, our innovative cancer detection technology has also begun to be recognized in other parts of Asia. Because our technology has many unique advantages (multi-cancer detection, low-cost cost, with good bioinformatics security, early detection capabilities, and high sensitivity and specificity) and consistency with local needs (these areas have huge market size and demand for cancer screening and risk assessment in the general population), we CDA technology is expected to have great revenue and development growth potential in the region. We are very satisfied with achieving this important milestone achievement, which shows that Anpac Biotech has entered the global equipment sales stage, which means that more and more experts and customers are accepting this new and great innovative detection method. With the continuous upgrading of people’s health needs, biophysical-based detection technology and its advantages are gradually being recognized and accepted by more and more customers and experts, and Gradually becoming a new force to promote the development of the industry. We will also work harder to uphold the beautiful vision of ‘keeping humans away from cancer’, adhere to the original intention of starting earlier and going further, and continue to develop innovative theories of multi-cancer screening and cancer treatment. Success in early screening.”


 < Online paper release screenshot >
< Online paper release screenshot > <CDA technical analysis Diagram >
<CDA technical analysis Diagram >


 The 113th American AACR Conference – Anpac Biotech
The 113th American AACR Conference – Anpac Biotech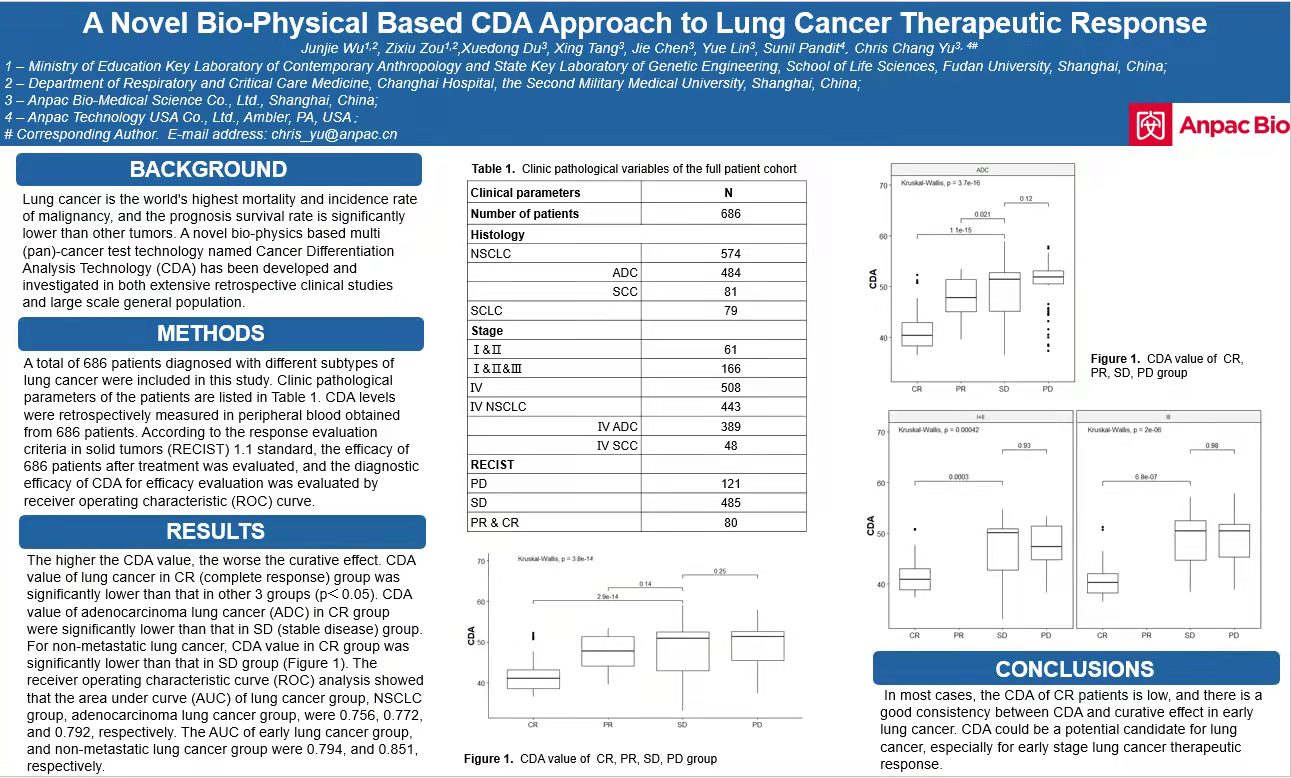

 Publish papers online
Publish papers online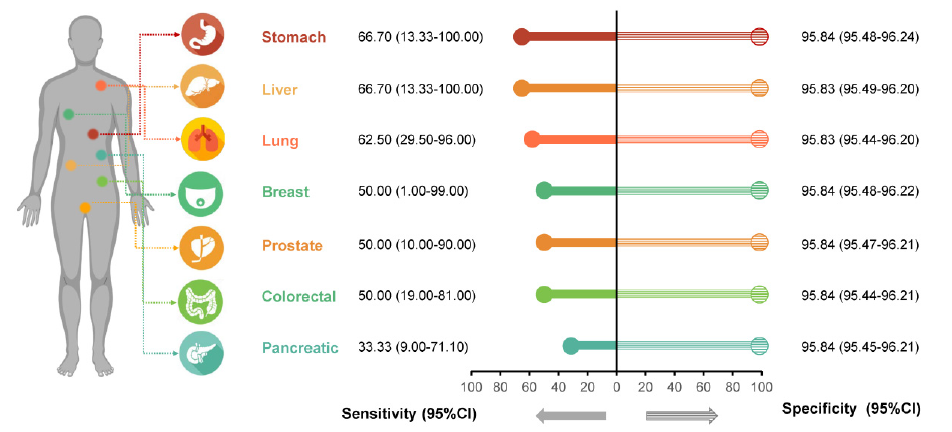 Application of CDA technology in follow-up (N=7999)
Application of CDA technology in follow-up (N=7999)
